Corrosion inhibitor and discoloration inhibitor for copper and copper alloy
TIME:2018-10-15CLICK:1526COME FROM:Los Copper, Copper Plate, Copper Strip, Copper Bar, Copper Tube, Copper Rod, Copper Foil, Copper Price, Haotai Copper
Corrosion inhibitor and discoloration inhibitor for copper and copper alloy
Properties,USES and methods of use of benzotriazole
The effect of air pollution on steel is well known.Recent problems of corrosion and discoloration of copper and copper alloys due to air pollution have also been highlighted.
As a method to prevent the corrosion and discoloration of copper and copper alloy,electroplating,coating,inhibitor and packaging can be cited.In this paper,the corrosion inhibition of styrene acrylic three?to prevent discoloration agent is introduced.
As inhibition of copper and copper alloy corrosion preventing discoloration agent,used for a long time.Consult?system and microphones?department and so on the many kinds of compounds,but in the***almost use the occasion of the"three benzene and?"is.However,due to the introduction of"three benzene and?"time is short,so the phenomenon is on the one hand,in the volatile corrosion inhibitor,oil,antifreeze solution,paint,etc have a wide range of USES,and not understanding of its properties.
Therefore,according to some data with the introduction of the"three benzene and?"were discussed.

1.Summary:
After 1.1 development:benzene and three?for the inhibition of copper and copper alloy corrosion preventing discoloration agent used started in 1960.As a water solvent can be seen sporadically in Europe and America.In the domestic 1962 Japan rust prevention technology association***times Europe and the United States rust prevention technology delegation to return to China,by the Japanese university yamamoto yoichi doctor introduced the literature and sample to carry on the industrial process test,the application of coating research.Generally in the oil,antifreeze and other aspects of the application.
1.2 name and method:
There are three kinds of name:1.2.3-benzene and three?(B.T)
Benzene and***AziminoBenzen
Benzene and***Benzen Azimide
The molecular weight is 119.1
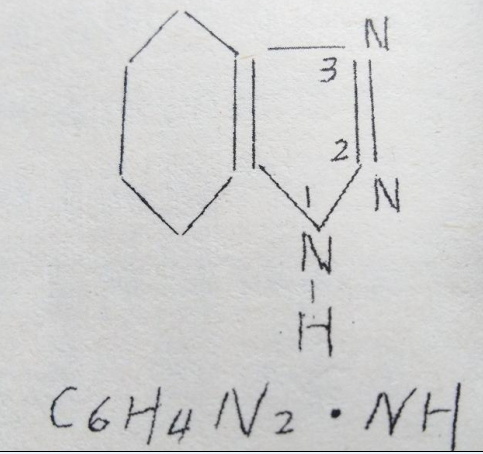
Structure:C6H4N2.NH
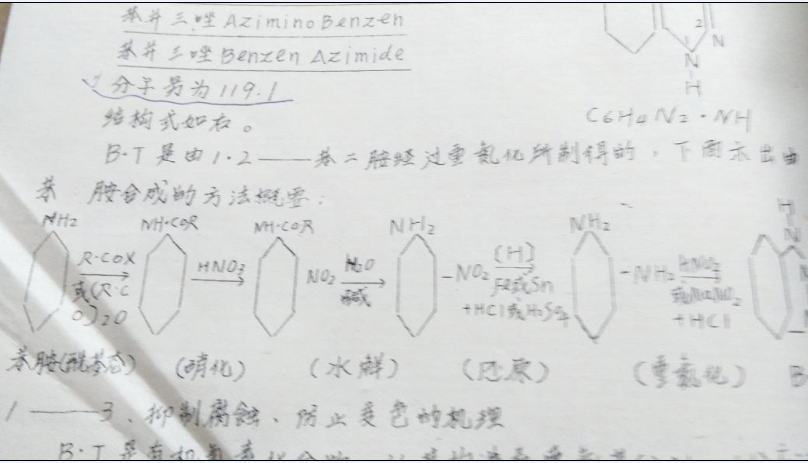
B.T is prepared from 1.2-phenylenediamine by diazotization.The method of synthesis from aniline is shown below
1.3 mechanism of inhibiting corrosion and preventing discoloration
B.T is an organic nitrogen compound whose structure of amino(>n-h)should be alkaline,but is actually weakly acidic in aqueous solution,which can be explained by the following dissociation formula.
C6H4N2.NH+H====C6H4N2N--
It is generally believed that the H of NH in this formula can be replaced with copper by the following reaction
Cu+1/2 o2+2 C6H4N2.NH===(C6H4N2.N)2 Cu+H2O
Through this displacement reaction,the"copper dibenzo***"molecular film insoluble in water is formed,which prevents the corrosion and discoloration of copper and copper alloy by blocking various corrosive environments.
Detailed experiments are being carried out with respect to these facts,but it is clear that B can be removed by simple solvent degreasing and cleaning,as well as by both anode and cathode suppression and by the coating treated with B.T.This could not be explained by the theory of chelate formation.
2.General shape:it is important to understand the nature of any drug when using it.Up to now,no detailed report has been published on the nature of B.T.The following is a comparison of B.T with the two compounds in the order of volatility,solubility,safety and toxicity.
2.1 volatility(sublimation)
The volatility of B.T is not only a difference from other inhibitors,but also an important characteristic.It means that B.T is applicable to the gas phase,that is,as mentioned later,it can be coated on paper,film and other materials for volatile corrosion inhibition.If the comparison is made at room temperature(25-30℃),the gasification force of DICHAN without steel is 1,then B.T gasification is 100.In addition,the gasification force of b.t.is 1/64 of naphthalene.

Table 1 vapor pressures of benzo***at various temperatures
Temperature(degree) | Benzene and***(mm hg) | Gasification rust inhibitor(mm hg) | Cyclohexamine carbonate Millimeters of mercury |
21 | - | 0.1 | - |
25 | - | - | 0.4 |
30 | 0.04 | 0.4 | 0.74 |
40 | - | 1.4 | 2.24 |
70 | 0.05 | - | - |
100 | 0.10 | - | - |
110 | 0.16 | - | - |
120 | 0.32 | - | - |
130 | 0.58 | - | - |
140 | 0.97 | - | - |
Table 2 solubility of benzo***(distilled water)
The temperature | The weight of the% | The temperature | The weight of the% |
10 | 1.19 | 45 | 5.30 |
15 | 1.38 | 50 | 5.66 |
20 | 1.57 | 60 | 6.54 |
25 | 1.96 | 65 | 7.41 |
30 | 2.34 | 70 | 8.59 |
35 | 2.91 | 80 | 10.46 |
40 | 3.85 | 90 | 15.25 |
Table 3 solubility of benzo**--(2)solvent(20℃)weight%
system | The name of the solvent | Benzene and*** | Gasification rust inhibitor* | Cyclohexamine carbonate* |
Polar solvent | methanol | 4.20 | 23.6 | 51.15 |
acetone | ﹥38.5 | 0.2 | 13.60 | |
Fibrinolytic agent(methyl,ethyl butyl) | 50.0 | |||
Heptyl acyl | 25.60 | |||
cyclohexanone | ﹥23.80 | |||
Ethylene glycol | 718.25 | 5.6 | ||
ether | 25.80 | 1.18 | ||
dioctylphthalate | 4.0 | |||
Chlorine is | trichloroethylene | 0.70 | 0.16 | |
perc | 0.30 | |||
Carbon tetrachloride | 0.16 | 0.006 | 3.55 | |
Oil system | benzene | 1.68 | 0.065 | 2.37 |
Oil solvent | 0.1 | |||
kerosene | 0.1 | |||
Spindle oil | 0.006 |
*
Solubility at 25℃
2.2 solubility
B.T is important for use in aqueous solutions or other solvents.Generally,it is well soluble in polar solvents,but it is difficult to be accommodated in water and petroleum solvents(refer to table 2 and 3).
2.3 stability
It is completely stable below 100℃in the atmosphere,but be aware that when heated sharply or in contact with red hot metals,toxic compounds like aniline and nitrobenzene are broken down.It is very stable to acid,alkali and oxygen reduction,but forms stable metal salts with alkali metals.Especially in acid and alkali stability this point,and the PH range of gasification rust inhibitor limited to 6.0-9.0,the application range is much broader.
Toxicity of 2.4
B.do not worry about toxicity in general use unless inhaled in large quantities.But it has a slightly bitter taste and irritates the mucous membranes of the nose.Therefore,it is recommended to use masks and other protection when handling large amounts of time.
As to virulent size,via the animal experiment that undertakes 20 days to white mouse,LD50(half lethal amount)=965 milligram/kilogram,namely every kilogram body weight is about 1 gram,it is vaporization sex to prevent rust agent and soda of nitrite,salicylic acid roughly 1/3.There is no hindrance in daily operation.
Table 4 results of oral injection of benzo***(comparison)
species | Lethal dose(20 day) |
The purity of benzo is 95% 98% | Median lethal dose=500 mg/kg Median lethal dose=937±28 mg/kg |
Compare gasification anti-rust agent Sodium nitrite | Median lethal dose=205±15 mg/kg Median lethal dose=220+57 mg/kg |
2.5 other properties
Pure B.T is a white crystalline powder,almost odorless,melted at about 95℃and sublimated at 98℃--100℃.The PH value of 0.1%aqueous solution is about 5.5--6.5.The purity sold in the general market is 95-98%(brown-white).When applied to a variety of transparent liquid systems,a 95%purity brown product can be colored fairly well by adding 0.5%,so use a high**color anyway.
3.Purpose and method of use
3.1 as a volatile material to prevent and inhibit corrosion and discoloration(VClS)
There has been a lot of literature about b.t.being a volatile corrosion-resistant agent to copper and copper alloys.There are methods for treating copper in b.t.saturated steam at 85℃and various packaging materials.
Column 1 b t 1 pa 50%solution 10 parts
Urea 12%90 parts
Sodium nitrite(NaNO2)12%
Hydroxydiethyl ammonium benzoate 24%
H2O 52%
Molecular formula:CONH3.CH2.(H2.OH)
For paper processing,if the amount of B.T is 5 g/m 2,the corresponding effect can be expected.
3.2 aqueous solution system
In the application of aqueous solution,generally divided into cooling water and antifreeze system anti-corrosion additives,and the use of B.T and other agents and pure B.T solution treatment of copper and copper alloy two categories.
(1)as a corrosion inhibitor
(2)as an antifreeze solution additive
(3)anti-discoloration treatment of primary products:it is easy to change from may to October in the plum rain season.Add 0.5%B.T into the hot water washing,the process after the desorption water washing after tempering,tempering and rolling,to prevent discoloration.
3.3 cleaning agent system
Generally,when using alkaline detergent on copper and copper alloy,if the PH exceeds 11.5,there will be a risk of corrosion and discoloration.In this case,if 0.5%b.t.B.T(r-bt)is added to the effective base component,it will have anti-corrosion effect.In addition,addition of 10--250PPm(0.001--0.025%)r-bt(R=C1~C16)to organic solvents(such as chlorinated solvents)or addition of B.T to abrasive may also be expected to prevent discoloration.
3.4 agent system
3.5 paint
B.T and its derivatives are applicable to copper and copper alloy coatings because it has the above effect of preventing copper from self-discoloration and secondary absorption of ultraviolet rays,that is,as uv absorbent,it has the effect of preventing degradation of the coating.
4.Other compounds
In addition to B.T,there are many compounds that are effective against copper and copper alloys.